Importing Medical Products for Personal Use in Japan: A Guide for Healthcare Professionals
Understanding the Regulatory Framework
Like many other countries, Japan regulates the importation of medical products—including pharmaceuticals, quasi-drugs, cosmetics, medical devices, and regenerative medicine products—to ensure public health and safety. These controls are in place to prevent the circulation of harmful or addictive substances and to maintain consistent safety standards across the healthcare system.
However, a complete ban on foreign-made products would limit access to potentially beneficial treatments. To address this, Japan has established an approval system through which medical products must be authorized before they can be marketed domestically.
Under this system, businesses that wish to distribute medical products in Japan must first obtain approval from the Ministry of Health, Labour and Welfare (MHLW). Additionally, each individual product must also receive approval before it can enter the domestic market.
The Exception: Personal Importation
An important exception exists for personal use. Individuals—including healthcare professionals—are allowed to import certain medical products for their own personal use without going through the full product approval process. This is referred to as personal importation.
Because these products are not reviewed through the formal MHLW approval process, individuals who import them bear full responsibility for their safe and appropriate use.
It is essential to note that personal importation is strictly limited to the importer’s own use. Giving, selling, or transferring these products to others—whether patients, friends, or third parties—is prohibited and considered a legal violation.
Trends in Personal Importation
Data from the MHLW shows that personal importation is common, especially among healthcare professionals.

In 2022, for example, over 200,000 products were imported under this framework, with the majority being pharmaceuticals and medical devices.
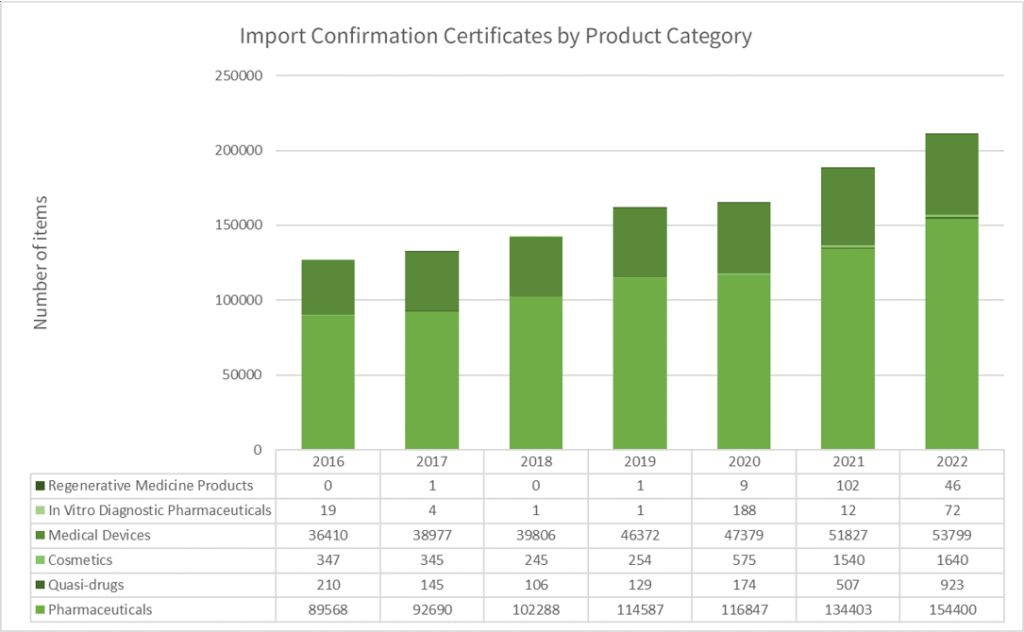
This growing trend reflects the needs of professionals who seek access to advanced or specialized products that are not yet available in Japan.
The Importation Process
o legally import medical products for personal use, you must obtain an Import Confirmation Certificate (Yunyu Kakunin-sho) from the relevant Regional Bureau of Health and Welfare (a regional administrative office under the Ministry of Health, Labour and Welfare).
The required documents may vary depending on the product category, intended use, and quantity. Below is a general example of what may be required:
- A completed import application form
- A detailed description of the product
- An invoice or proof of purchase
- A copy of your medical license (for healthcare professionals)
- A statement explaining the necessity of the product and lack of domestic alternatives
- Shipping documents such as an air waybill or tracking number
In some cases, online application may be available depending on the type of product and procedure. Please check the official website of the applicable Regional Bureau for the latest instructions and forms.
Support from Gyoseishoshi – Certified Specialist
For busy healthcare professionals, managing the application process can be time-consuming and complex. Administrative scriveners (Gyoseishoshi) are certified legal specialists in Japan who can assist with preparing and submitting documents for personal importation and regulatory procedures.
If you need help navigating the requirements or want to delegate the paperwork, feel free to contact our office. We provide reliable support to ensure your import process is smooth, compliant, and stress-free.
